Clinical Trial to Evaluate a New System for Patients with Irregular Heartbeats
Houston, TX (March 10, 2020) — The Texas Heart Institute is participating in a multi-center clinical trial to assess the safety and effectiveness of a new system that could help nearly 1.5 million heart failure patients around the world suffering from their heart beating out of sync.
The WiSE Cardiac Resynchronization Therapy (CRT) System is being evaluated in clinical trials in the US, Europe, and Australia. The system includes a tiny investigational device that delivers left ventricular pacing to the heart for patients in need of CRT but whose irregular heartbeat has not responded to conventional forms of CRT or to other therapies.
 © 2017 EBR Systems, Inc. All rights reserved. Used with permission by EBR Systems, Inc
© 2017 EBR Systems, Inc. All rights reserved. Used with permission by EBR Systems, Inc
Heart failure is a serious condition in which the heart is unable to pump enough blood to meet the body’s demands. Heart failure often occurs when electrical signals within the heart are disrupted, causing the heart’s ventricles to beat in an uncoordinated or unsynchronized pattern, which in turn enlarges the left ventricle and makes the heart less efficient.
For many, medicine only helps to a degree, which is why their doctor recommends CRT. Typically, this involves implanting a pacemaker to stimulate the heart electrically. While standard pacemakers can correct an irregular heartbeat, CRT devices are responsible for making sure the entire heart beats synchronously.
CRT is also referred to as “biventricular pacing” because it uses wire leads to synchronize the left and the right ventricles so that the two chambers beat together— This improves the heart’s efficiency.
Studies have demonstrated that successful CRT therapy improves symptoms and reduces hospitalizations and mortality. About 200,000 patients are treated worldwide each year with traditional CRT, and about 155,000 of them are in the U.S. However, traditional CRT has limitations that prevent some patients from benefiting.
In a traditional CRT procedure, a pacemaker is implanted under the skin. Two wires, referred to as leads, are typically placed inside the right heart: one in the right atrium and one in the right ventricle. A third lead is placed in a vein called the coronary sinus on the outside of the left ventricle. The leads carry electrical pulses to synchronize the heartbeat. Approximately 30% of patients who receive a traditional CRT device do not benefit and are classified by physicians as “non-responders.”
“Unfortunately, traditional CRT devices do not always work for patients,” says Dr. Nilesh Mathuria, Principal Investigator of the study. “The coronary sinus vein is like a roadway system that only goes to certain locations on the outside of the heart. Thus, we do not have a complete choice of where to place the pacing lead to best stimulate the heart. CRT devices also may not work because they can’t be placed, or they may stimulate nerves in a way that bothers the patient.”
These problems, along with adverse events associated with CRT, have placed a substantial burden on the healthcare system, including the need for repeated surgeries and hospitalizations for heart failure.
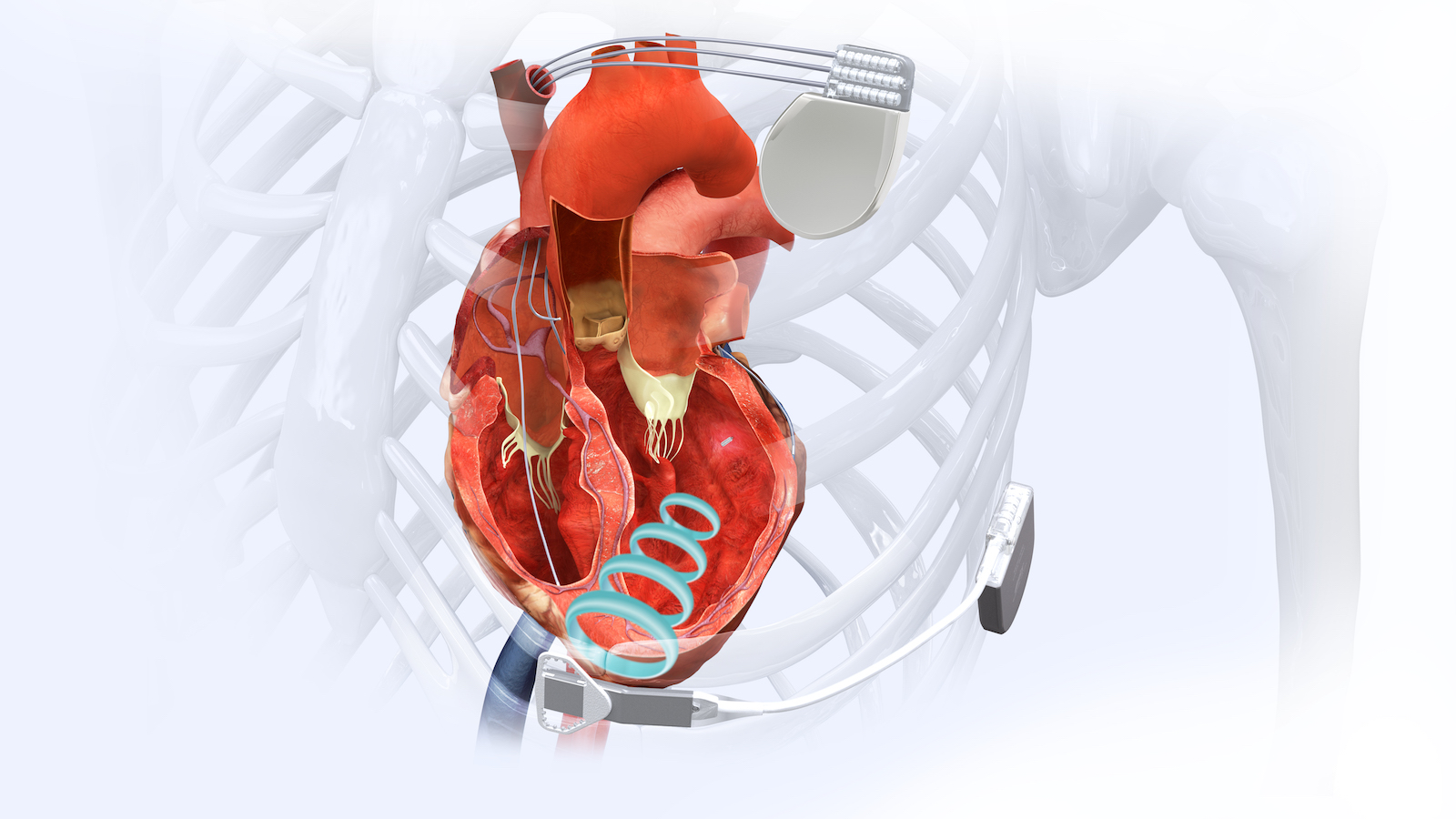
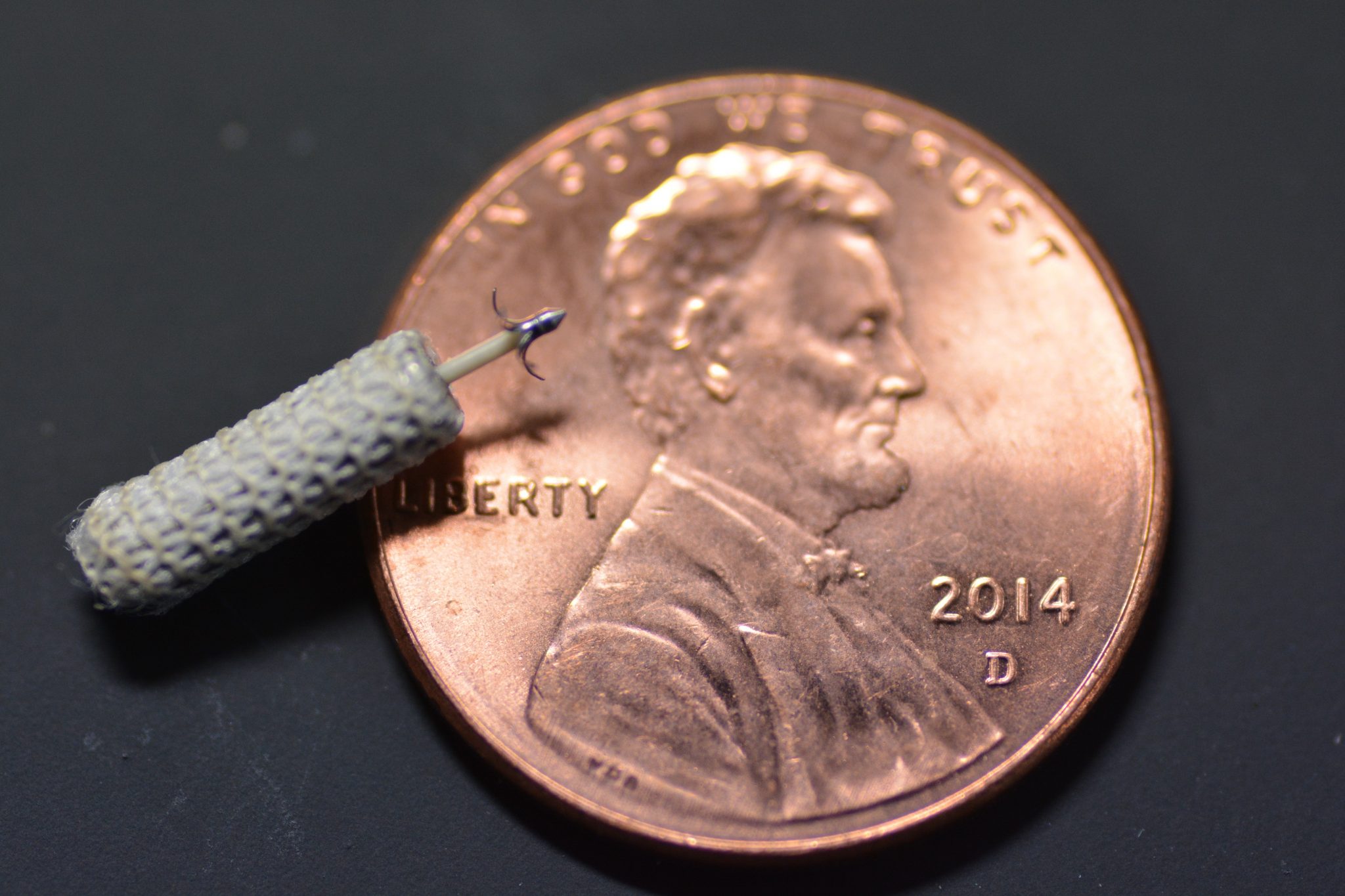
The WiSE CRT System is a first-of-its-kind device designed to overcome the limitations of traditional CRT in heart failure patients. Instead of using pacing leads—decades-old technology with well-documented problems—the WiSE system uses a small, wireless electrode the size of a large grain of rice. Via a catheter, the wireless electrode is implanted directly at a customized location inside the heart’s left ventricle, where protective endothelial cells quickly grow over the electrode’s polyester scaffolding. An innovative wireless technology is used to deliver pacing stimulation through the electrode to the inside of the heart’s left ventricle, referred to as endocardial stimulation. In fact, the name “WiSE” stands for “Wireless Stimulation Endocardially.” The system works together with a previously implanted pacemaker or implantable cardioverter-defibrillator that paces the right side of the heart. The WiSE CRT System paces the left side at the same time as the right side to potentially improve the heart’s pumping ability and help overcome symptoms of heart failure.
The WiSE CRT System is being studied in a clinical trial called SOLVE-CRT, which is enrolling 350 patients at up to 45 active U.S. and international investigational sites. The WiSE CRT System is investigational in the United States and is not yet commercially available; no claims of safety or efficacy can be made until the study completes. Study completion and U.S. FDA approval for the WiSE CRT System are expected in 2020.
To learn about the study, contact the Texas Heart Institute Center for Clinical Research at clinicalresearch@texasheart.org or Phone 832-355-9405.
#
About Texas Heart Institute
The Texas Heart Institute (THI), founded in 1962 by world-renowned cardiovascular surgeon Dr. Denton A. Cooley, is a nonprofit organization dedicated to reducing the devastating toll of cardiovascular disease through innovative and progressive programs in research, education, and improved patient care. More information about THI (@Texas_Heart) is available at www.texasheart.org.
Media Contact
Keri Sprung
Texas Heart Institute
ksprung@texasheart.org
832 355-9591
About EBR Systems and The WiSE CRT System
EBR Systems, Inc. a Silicon Valley California based company, is driven to deliver superior treatment for millions of patients suffering from cardiac rhythm diseases by developing safe, clinically superior, cost- effective, and reliable therapies using wireless cardiac stimulation. The company’s initial product, the, is a first-of-its-kind medical device developed to overcome the limitations of traditional cardiac resynchronization therapy (CRT) in heart failure patients by eliminating the need for a lead to the heart’s left ventricle and the complications associated with that lead. Future products will address wireless endocardial stimulation for bradycardia and other non-cardiac indications.




